| Enterprise Solutions | Instrument Services | Parts & Consumables | Support | Training | About Us | Contact Us |
USA phone for analytical instruments: 888-860-5227, Option 3
USA phone for laboratory equipment: 1 888-860-5227, Option 3, then 1
Need contact info for other countries? Click here.

Did you say Pareto Charts?
By Kelly Huckabone
June 2020
The Pareto Chart analyzes the frequency of problems or causes in a process. In quality control, it most often represents the most common sources of defects, the highest occurring type of defect, or the most frequent reasons for why something is happening.
We use a Pareto Chart as a key tool when working through practical process improvement (PPI) or Lean projects. Typically, you start with a larger amount of data and after sorting it into “buckets,” an idea of where a deeper dive may be required should be apparent.
Data from a Pareto Chart can help you assess or validate the magnitude of the issue and then help you narrow down areas of focus. This is the basic concept of the 80/20 rule in that roughly 80 percent of the effects come from 20 percent of the causes. Here are the basic steps:
Step 1
Develop a list of problems, items, or causes to be compared.
Step 2
Develop a standard measure for comparing the items (frequency, time, and cost).
Step 3
Choose a timeframe for collecting the data.
Step 4
For each item, tally how often it occurred (depending on what you are assessing).
Step 6
List the items from highest to lowest on the horizontal axis of a graph. Label the left vertical axis with the numbers (frequency, time, or cost).
Step 7
Analyze the diagram by identifying those items that appear to account for most of the difficulty. The next step will typically involve performing a next-level Pareto for the most commonly occurring data.
Here is an example of a Pareto Chart from a past project we worked on:
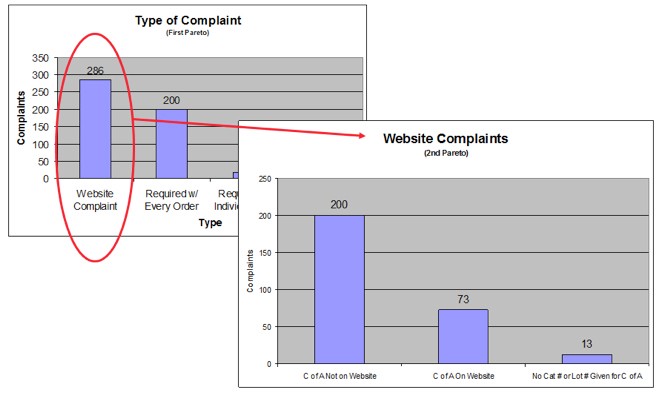
The Pareto Chart is a visual tool that can help a team discover where to focus attention when seeking the root cause of a problem.
Download a PDF of this article

About the author
Kelly Huckabone is the North American Audit Program Manager who oversees the Unity™ Lab Services internal and external customer and supplier audit programs. Kelly is a certified risk manager, lead auditor with the American Society for Quality (ASQ), and has been conducting audits for over 25 years for different quality systems, including ISO 9001, 13485, and 17025, as well as Health Canada and the FDA.
Contact me at kelly.huckabone@thermofisher.com if you have any questions.